

KEY DATA
Client: Novartis
Location: Austria
Year: 2019
Size: 5.000 m2 (gross surface)
The need
Realize savings by concentrating QC testing operations for biological products in one single location and design a lab that can accommodate the expected future capacity required by the biologics market.
The solution
Design and build a new lab building with approximately 5.000 m2, including analytical chemistry, microbiology and qPCR labs. The new building would be integrated in an existing site and following the directives set fourth by the site master plan.
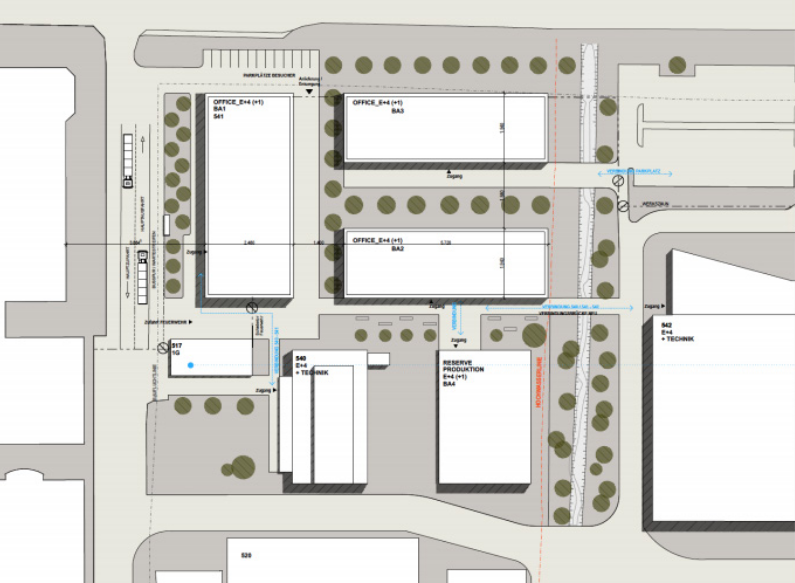


What we did and how we did it
We developed the concept and basic design for the new lab, interacting closely with the end users of each of the labs, having as main objective the definition of the best work environment for lab that would support the manufacturing units in the years to come.
Efficiency of operations, capital investment and future proofing were at the core of the work developed, which culminated in a building design that matched the client expectations and needs. The use of the latest design tools guaranteed that the design intent was correctly conveyed and the end user understood early on in the process what the end result would be.



About
Novartis is an innovative medicines company. The company focuses on core therapeutic areas with high unmet patient needs such as cardiovascular, renal and metabolic, immunology, neuroscience and oncology. Novartis utilizes technology platforms that enable cutting-edge therapies such as chemistry, biotherapeutics, xRNA, radioligand therapy and cell & gene therapy.



